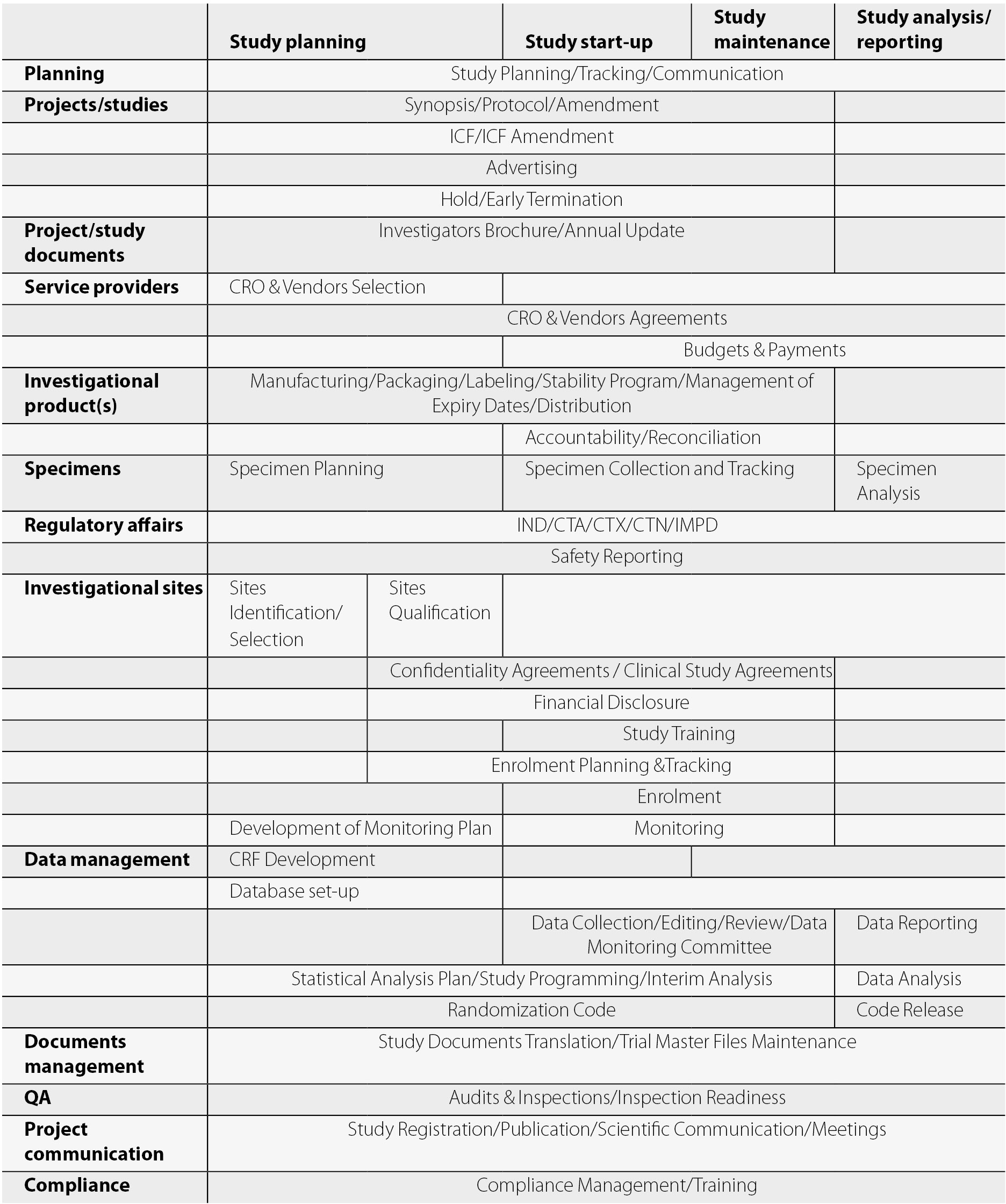
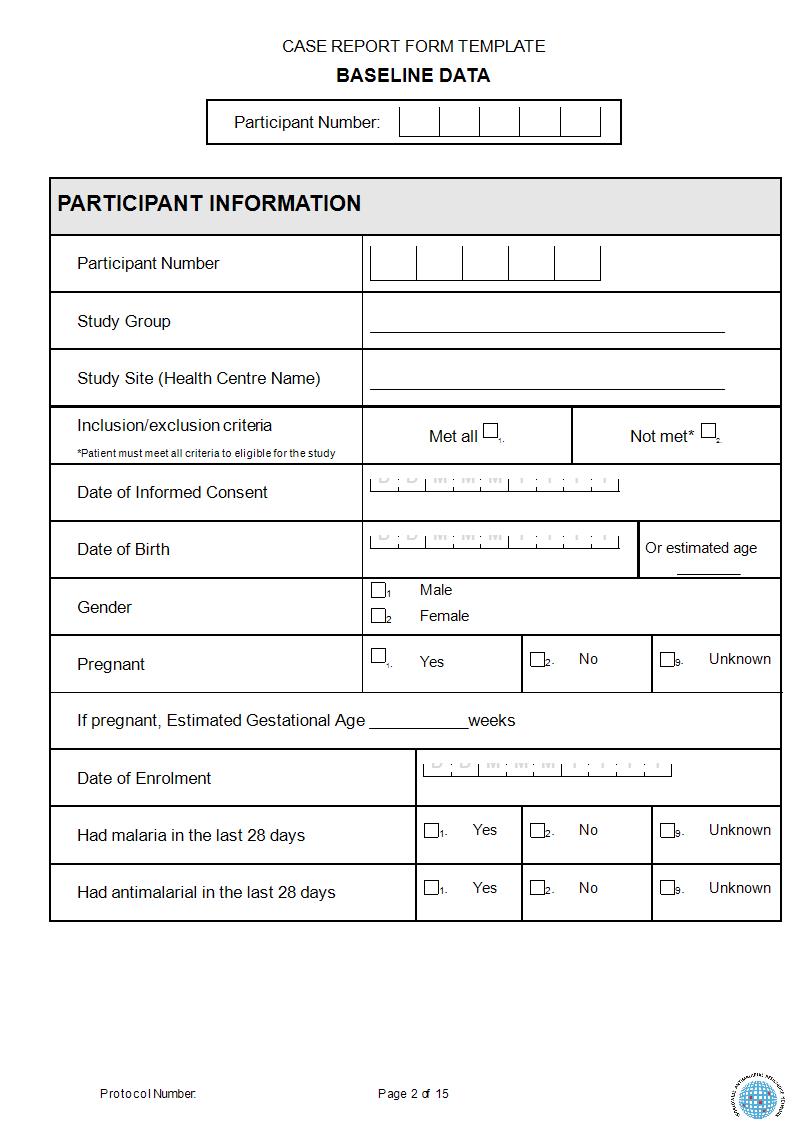
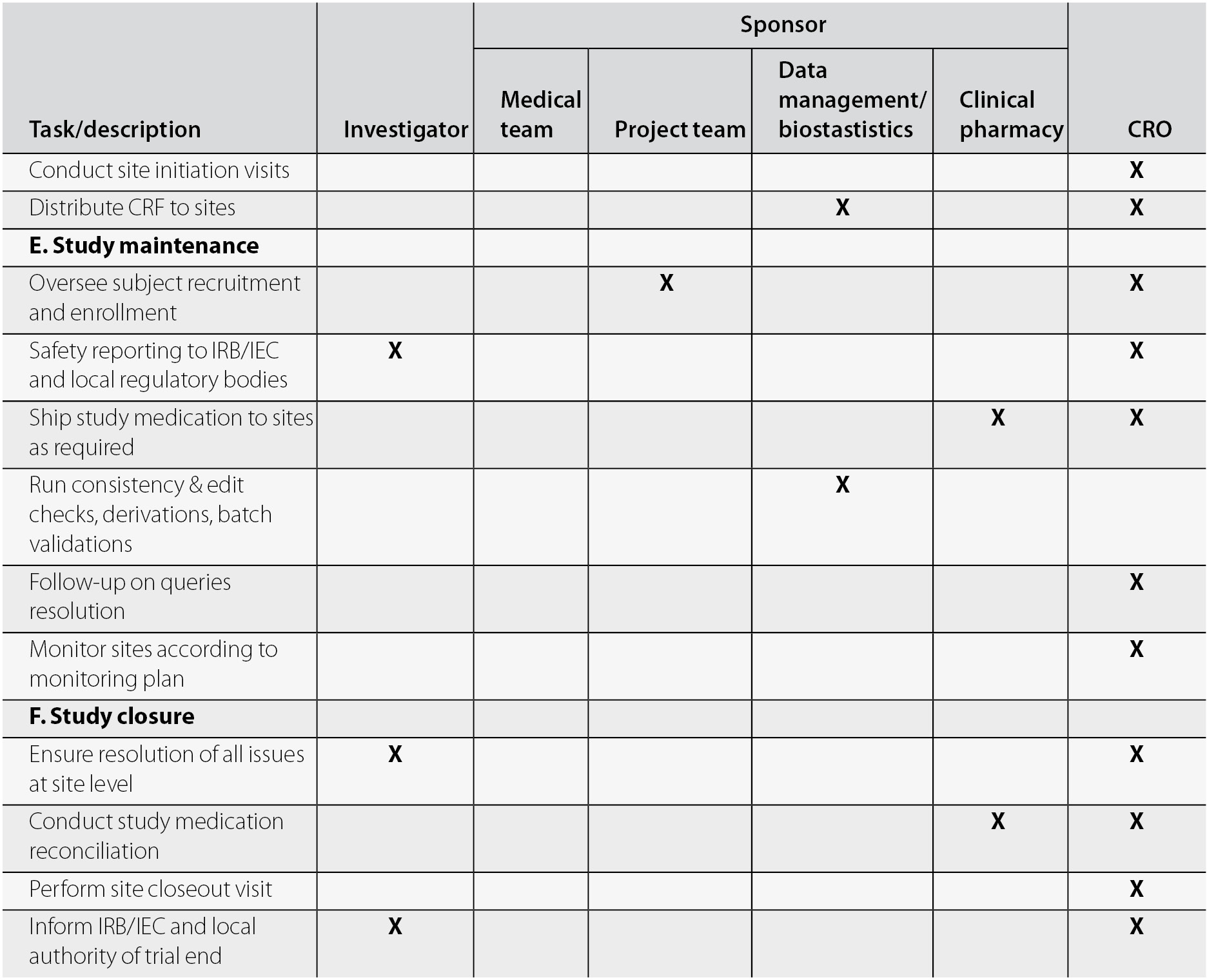
Clinical Trials Support Unit task delegation log. AE, adverse event;... | Download Scientific Diagram

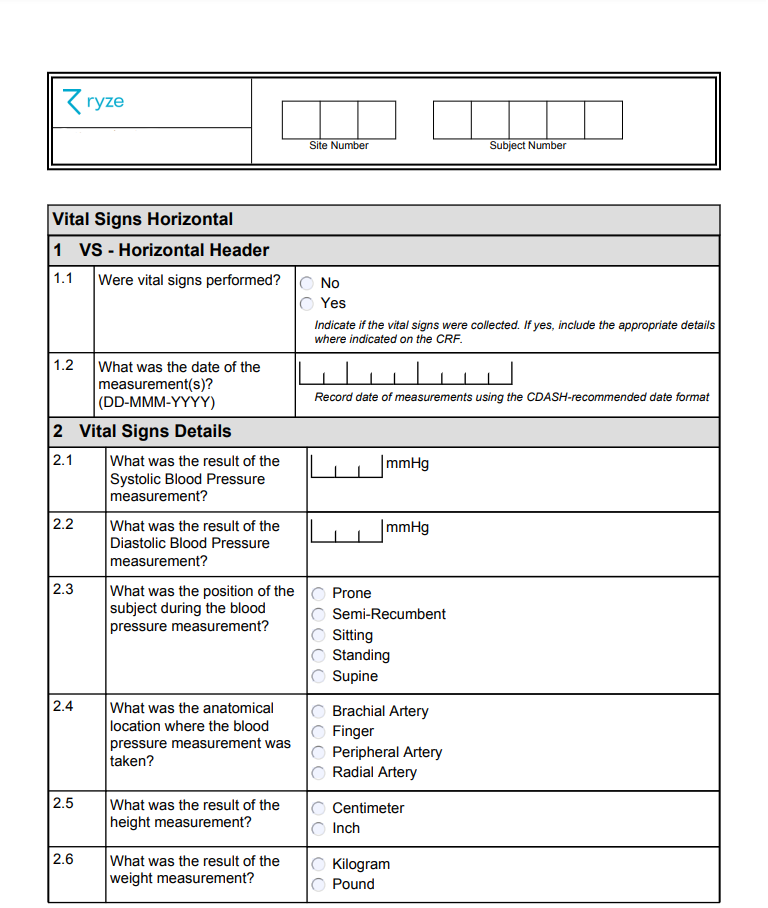
Case Report Form Template Clinical Trials (5) - TEMPLATES EXAMPLE | TEMPLATES EXAMPLE | Clinical trials, Clinic, Clinical research

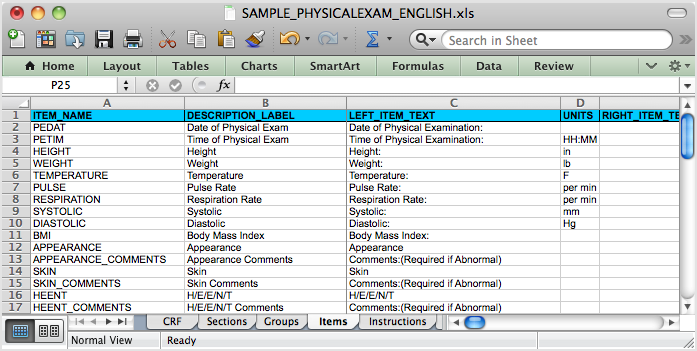
Case Report Form Template Clinical Trials (3) - TEMPLATES EXAMPLE | TEMPLATES EXAMPLE | Report template, Study site, Clinical trials

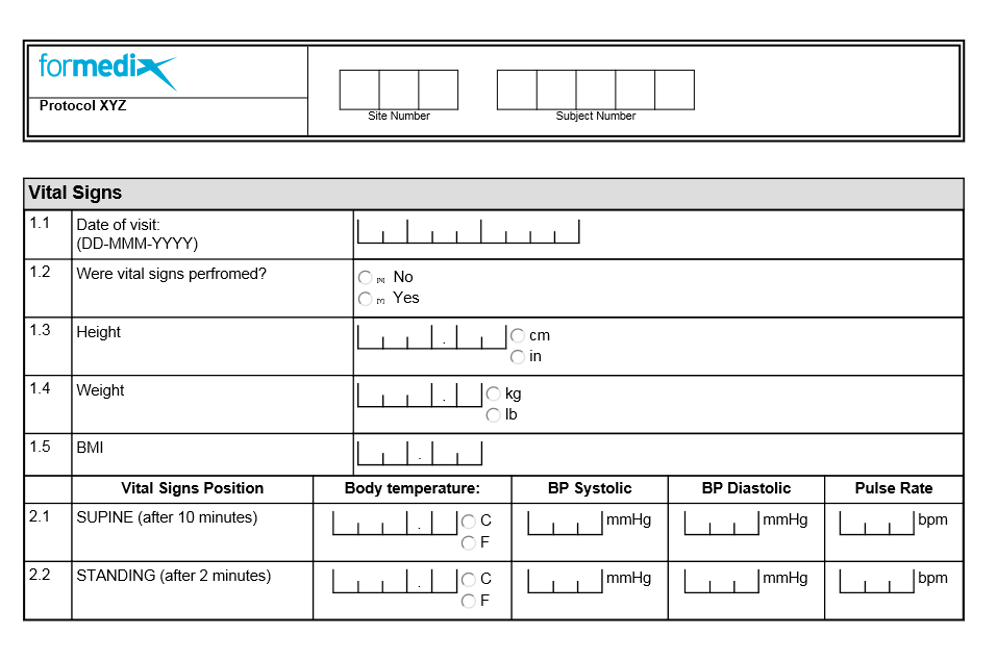
Long-term follow-up form. Simple one page CRF for collection of primary... | Download Scientific Diagram