A randomised, placebo-controlled phase 3 study to evaluate the efficacy and safety of ASP0113, a DNA-based CMV vaccine, in seropositive allogeneic haematopoietic cell transplant recipients - eClinicalMedicine
Vaccine therapy for cytomegalovirus in the setting of allogeneic hematopoietic cell transplantation: Expert Review of Vaccines: Vol 14, No 3

A randomized, phase 2 study of ASP0113, a DNA‐based vaccine, for the prevention of CMV in CMV‐seronegative kidney transplant recipients receiving a kidney from a CMV‐seropositive donor - Vincenti - 2018 -
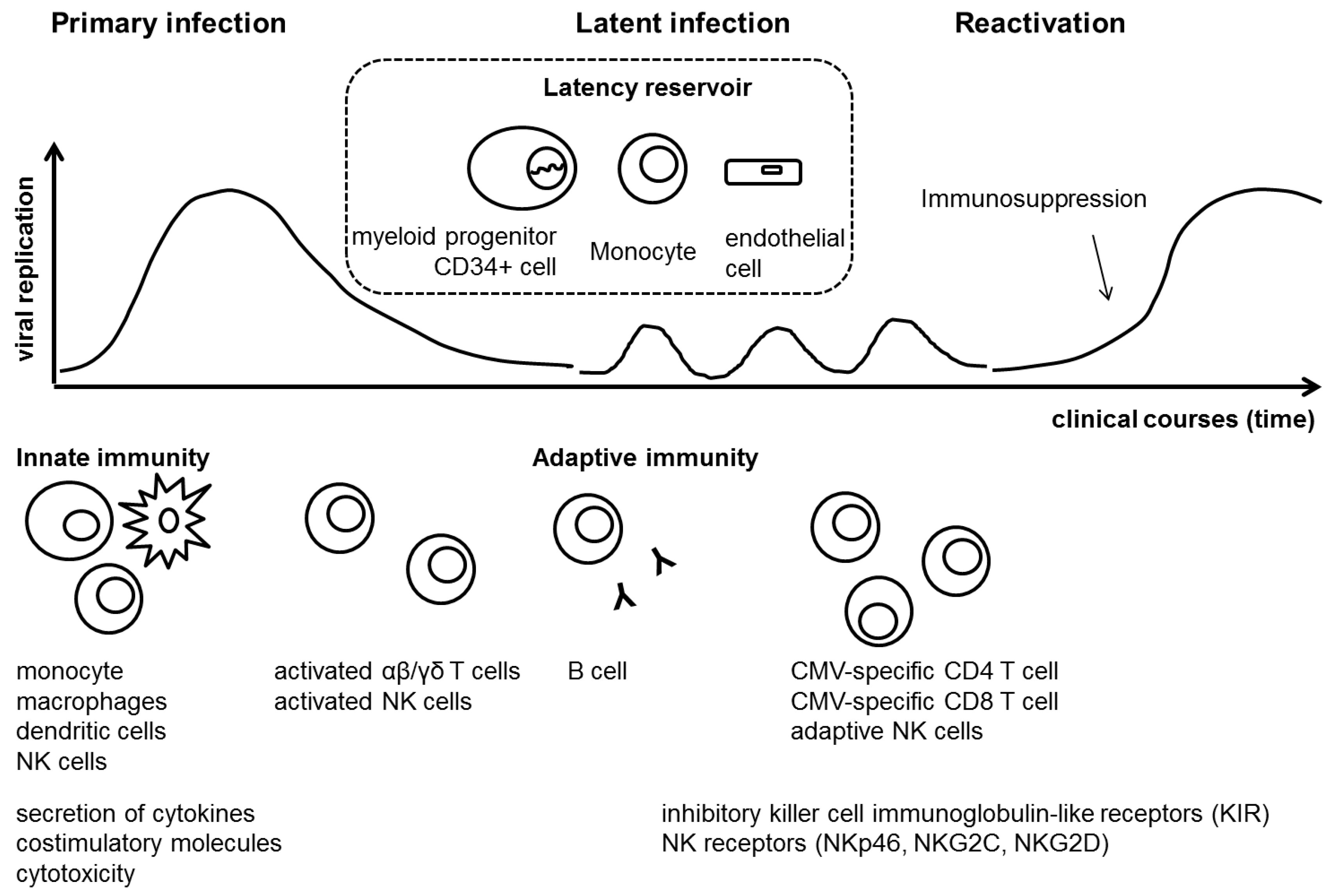
IJMS | Free Full-Text | Cytomegalovirus Infections after Hematopoietic Stem Cell Transplantation: Current Status and Future Immunotherapy | HTML
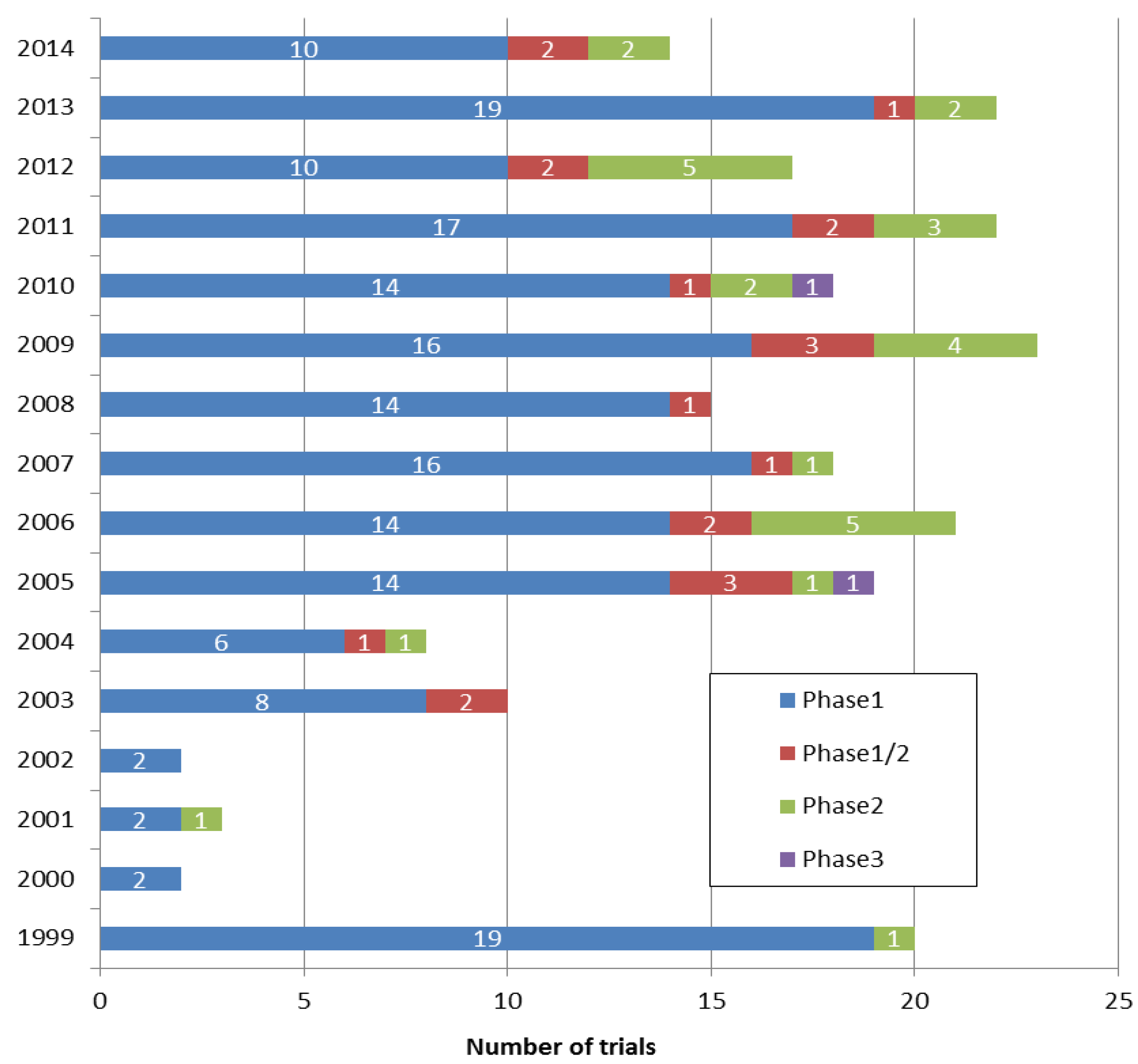
Vaccines | Free Full-Text | Comparison of Current Regulatory Status for Gene-Based Vaccines in the U.S., Europe and Japan | HTML

A randomised, placebo-controlled phase 3 study to evaluate the efficacy and safety of ASP0113, a DNA-based CMV vaccine, in seropositive allogeneic haematopoietic cell transplant recipients - eClinicalMedicine

A randomised, placebo-controlled phase 3 study to evaluate the efficacy and safety of ASP0113, a DNA-based CMV vaccine, in seropositive allogeneic haematopoietic cell transplant recipients. - Abstract - Europe PMC
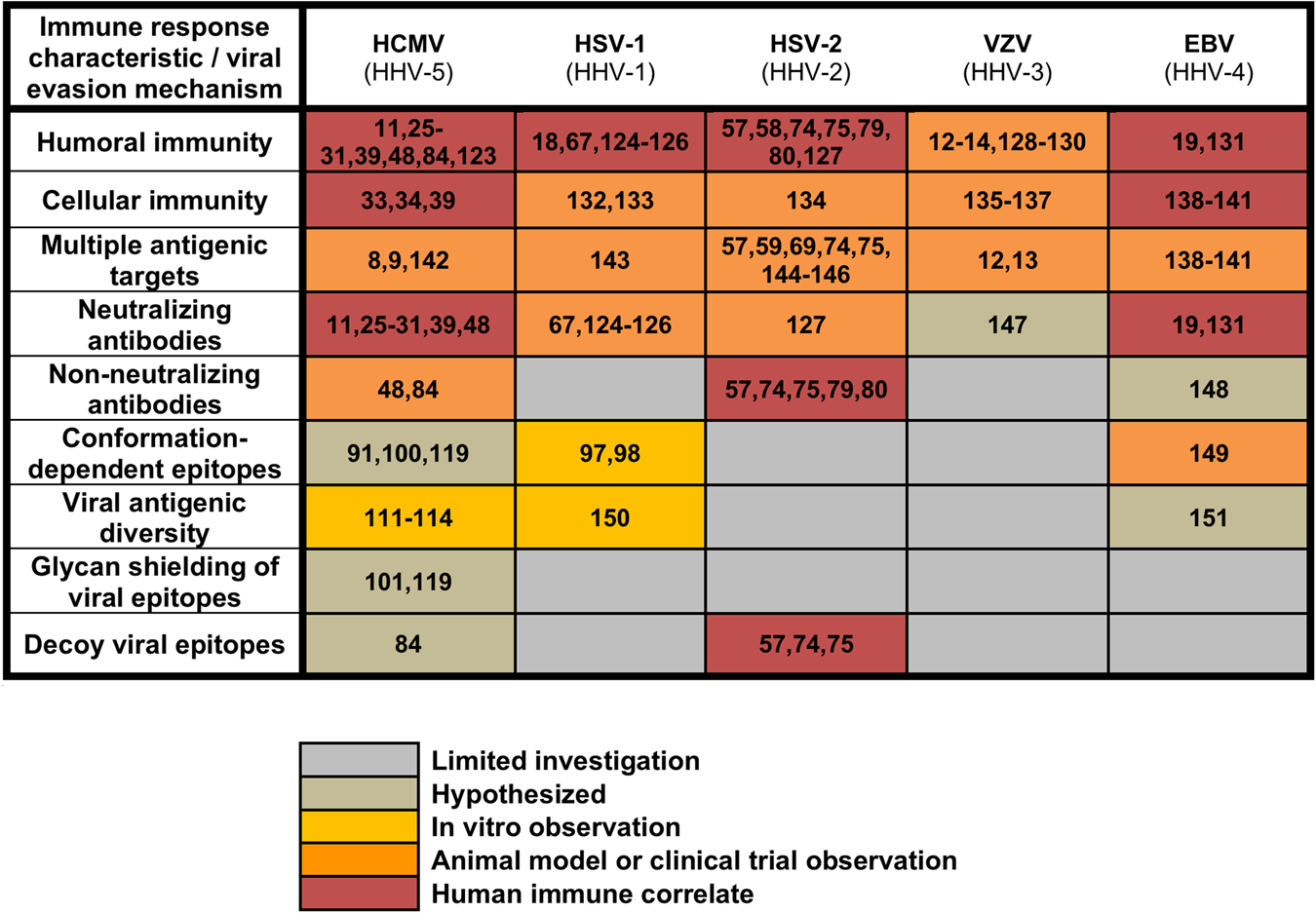
A new era in cytomegalovirus vaccinology: considerations for rational design of next-generation vaccines to prevent congenital cytomegalovirus infection | npj Vaccines

A randomised, placebo-controlled phase 3 study to evaluate the efficacy and safety of ASP0113, a DNA-based CMV vaccine, in seropositive allogeneic haematopoietic cell transplant recipients - eClinicalMedicine
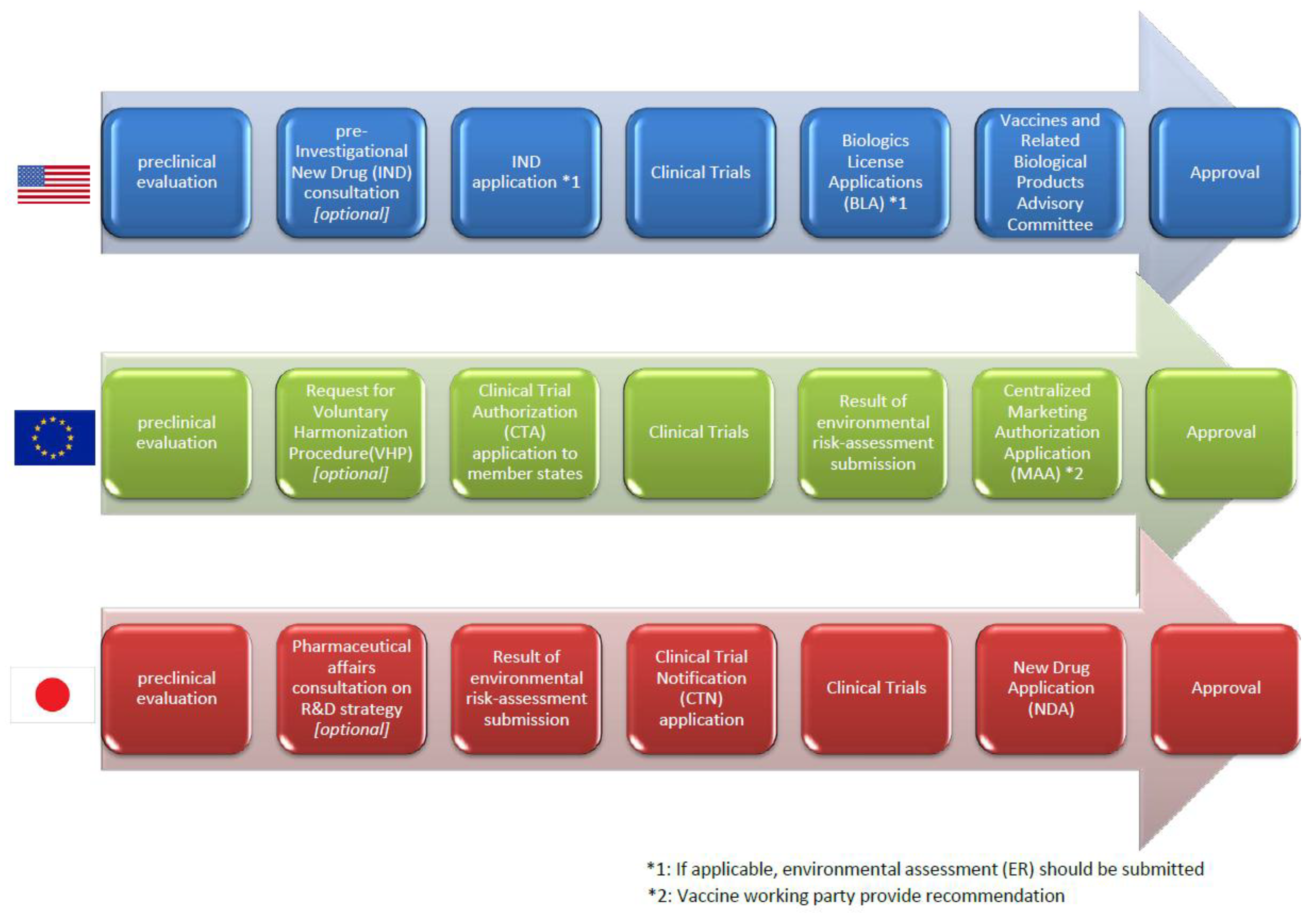
Vaccines | Free Full-Text | Comparison of Current Regulatory Status for Gene-Based Vaccines in the U.S., Europe and Japan | HTML

A randomised, placebo-controlled phase 3 study to evaluate the efficacy and safety of ASP0113, a DNA-based CMV vaccine, in seropositive allogeneic haematopoietic cell transplant recipients - eClinicalMedicine

PDF) A randomised, placebo-controlled phase 3 study to evaluate the efficacy and safety of ASP0113, a DNA-based CMV vaccine, in seropositive allogeneic haematopoietic cell transplant recipients
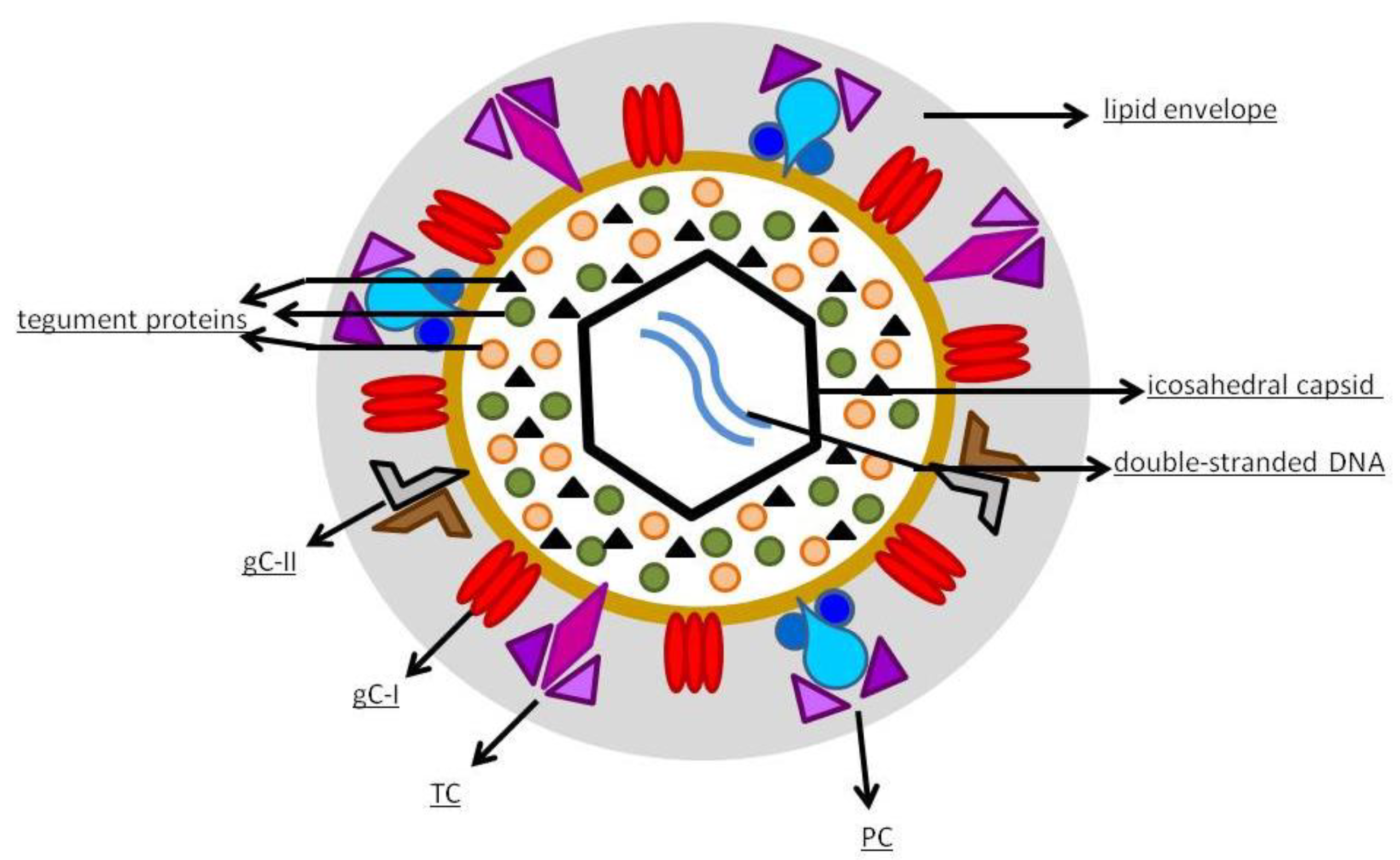
Vaccines | Free Full-Text | Development of a Vaccine against Human Cytomegalovirus: Advances, Barriers, and Implications for the Clinical Practice | HTML

A randomised, placebo-controlled phase 3 study to evaluate the efficacy and safety of ASP0113, a DNA-based CMV vaccine, in seropositive allogeneic haematopoietic cell transplant recipients - eClinicalMedicine

Vical's Astellas-partnered CMV vaccine falls short again, this time in stem cell transplant recipients | Fierce Pharma